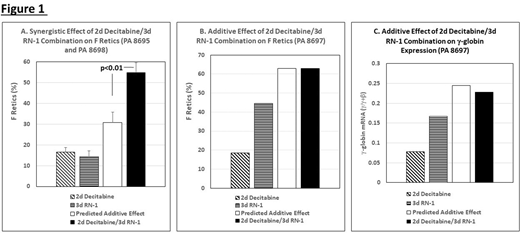
Sickle cell disease (SCD) is an inherited blood disorder that severely impacts the quality of life and reduces the life expectancy of approximately 100,000 patients in the U.S. and millions worldwide. Elevated levels of Fetal Hemoglobin (HbF; α2γ2) reduce the severity of symptoms and lengthen the life span of patients with sickle cell disease by inhibiting deoxy HbS polymerization. Hydroxyurea (HU), the only FDA-approved drug shown to increase HbF, is not effective in a subset of patientsand importantly, in the refractory individuals the increased HbFis heterogeneously distributed within the erythrocyte population resulting in a large fraction of erythrocytes lacking protective levels. Simian primates such as the baboon are widely acknowledged as the best animal models for testing the ability of new drugs to increase γ-globin expression because results in baboons are predictive of effects in man due to conservation of the structure and developmental stage-specific regulation of the β-like globin genes in simian primates. Our previous studies in baboons have shown that the most powerful drugs for reactivation of HbF are DNMT1 and KDM1A inhibitors targeting enzymes located within the co-repressor complexes that act to establish and maintain repressive epigenetic modifications at the γ-globin promoter to repress γ-globin expression. Treatment of baboons with either decitabine, an inhibitor of DNMT1, or RN-1, an inhibitor of KDM1A, strongly stimulates HbF. Dose-limiting effects of these drugs include neutropenia, thrombocytopenia, and/or thrombophilia. In this investigation we have explored the hypothesis that a treatment regimen comprised of both decitabine and RN-1 could achieve additive or synergistic effects on HbF while minimizing adverse hematological side effects. Three baboons (PA8695, PA8697, PA8698) were treated with two courses of different doses of RN-1 (0.25 and 0.125mg/kg/d) administered subcutaneously for 3 days to select an optimal RN-1 dose for each animal. The response to RN-1 varied between individual animals and was associated with pretreatment levels of γ-globin expression. Higher pretreatment levels of γ-globin expression were observed in PA8697 (0.08 γ/γ+β) compared to PA8695 (0.014+0.005 γ/γ+β) and PA8698 (0.004+0.0007 γ/γ+β) and the effect on γ-globin expression in PA8697 (0.24+0.5 γ/γ+β) at the 0.125mg/kg/d dose was greater than the effect of 0.25 mg/kg/d dose in PA8695 (0.17+0.05 γ/γ+β) and PA8698 (0.121+0.006 γ/γ+β). For combinatorial studies with decitabine, an RN-1 dose of 0.125mg/kg/d was chosen for PA8697 and 0.25mg/kg/d for PA8695 and 8698. Decitabine (0.3mg/kg/d; 2d), administered as a single agent subcutaneously for two days increased F retics nearly 3 fold (23.6+12.5%; p<0.02) compared to pretreatment levels (8.26+9.90%) but changes in γ-globin expression (0.066+0.076 γ/γ+β) compared to the pretreatment levels (0.03+0.042 γ/γ+β) were not significant. For combinatorial experiments, decitabine (0.3mg/kg/d) was administered for 2 days followed by administration of RN-1 (0.25mg/kg/d PA8695 and 8698; 0.125mg/kg/d PA8697) for 3 days. This staggered dose schedule was used to avoid any possible effects of RN-1 on cell cycle kinetics that could potentially reduce incorporation of decitabine during S phase. The decitabine/RN-1 combination produced significantly higher increases in F retics (63.0+23.6%; p<0.002) than either RN-1 (29.9+27.1%) or decitabine (23.6+12.5%) alone. The effect of the decitabine/RN-1 combination on F retics was greater than additive (p<0.01; Figure 1A) in the two low HbF animals, PA8695 and 8698, and additive for the high HbF animal PA8697 (Figure 1B). Although increased levels of γ-globin expression produced by the decitabine/RN-1 combination (0.22+0.08 γ/γ+β) were not significantly different overall from those induced by RN-1 alone (0.178+0.06 γ/γ+β), individual analysis of PA8697 revealed an additive effect (Figure 1C). Plt and ANC remained with the normal range. In conclusion, targeting separate components of the γ-globin co-repressor complex by combinatorial administration of decitabine and RN-1 generated synergistic and additive increases in F retics and γ-globin expression in the absence of hematological toxicity. Future experiments will explore different doses, alternative schedules, and the effect of longer term treatment regimens.
Saunthararajah:EpiDestiny: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal